products categories
- Battery Production Equipment Line
- Battery Lab Pilot Equipment Line
- Lithium Battery Pack Assembly Line
- Sodium Ion Battery Production Line
- Solid State Battery Assembly Line
- Supercapacitor Assembly Line
- Lithium Ion Battery Recycling Plant
- Dry Electrode Preparation Solution
- Li ion Battery Materials
- Cathode Active Materials
- Anode Active Materials
- Customized Battery Electrode
- Coin Cell Parts
- Lithium Chip
- Cylindrical Cell Parts
- Battery Current Collectors
- Battery Conductive Materials
- Electrolyte
- Metal Mesh
- Battery Binder
- Separator and Tape
- Aluminum Laminate Film
- Nickel Strip
- Battery Tabs
- Graphene Materials
- Titanium Fiber Felt
- Battery
- Battery Pack Machine & Compoments
- Lithium Battery Machine
- Battery Tester & Analyzer
- Battery Safety Tester
- Battery Material Tester
- Rolling Press Machine
- Spot Welding Machine
- Vacuum Mixer Machine
- Crimping/Disassembling Machine
- Vacuum Sealing Machine
- Electrolyte Filling
- Stacking/Winding Machine
- Electrode Cutter/Slitter
- Pouch Forming Machine
- NMP Solvent Treatment System
- Lithium Battery Production Plant
- Vacuum Glove Box
- Furnaces
- Coaters
- Hydraulic Press
- Ball Mill
- Planetary Centrifugal Mixer
- Laboratory Machine
- Metal Foam
contact us
- If you have questions, please contact us, all questions will be answered
- WhatsApp : +86 13003860308
- Email : David@tmaxcn.com
- Email : Davidtmaxcn@gmail.com
- Add : No. 39, Xinchang Road, Xinyang, Haicang Dist., Xiamen, Fujian, China (Mainland)
2021-07-10
When you need a mobile phone most, are you worried that it won't work? What do you do when you drive an electric car and there is no power in it? For these questions, light lithium sulfur battery can answer for you! Its energy storage capacity is more than twice that of batteries on supermarket shelves, but it usually has no electricity and short service life. Scientists at the joint research center for energy storage research and the Pacific Northwest National Laboratory have found one of the reasons behind this problem.
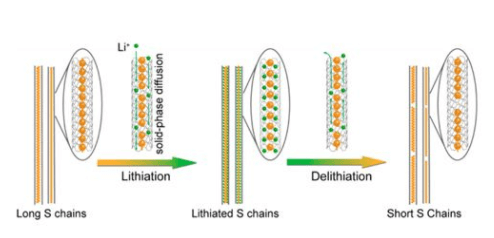
They found that the salt used in the electrolyte in the battery makes a big difference. When a salt called LITFSI lithium trifluoromethylsulfonimide is used as the electrolyte of the battery, lithium battery assembly equipment can test the battery for more than 200 charging and discharging operations. In lithium sulfur battery, lifsi combines lithium and sulfur atoms on the electrode, but it is released quickly. On the contrary, similar electrolytes have stronger binding force to lithium and sulfur atoms, and do not release at all. The performance of the cell decreases rapidly, and the battery has no energy after running for dozens of times.
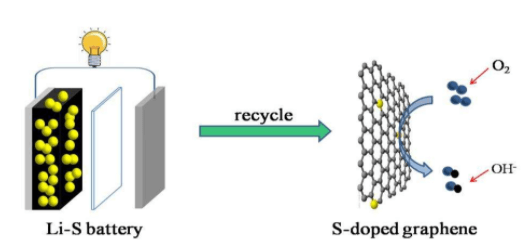
One of the problems with first mover vehicles is that drivers are trapped between charging stations for a long time. This concern led consumers to decide to buy low emission cars. The results of this study add another important factor to the design of high energy lithium sulfur battery.

 Cindy@tmaxcn.com
Cindy@tmaxcn.com David@tmaxcn.com
David@tmaxcn.com +86 13003860308
+86 13003860308 18659217588
18659217588